Baxter Prismaflex Ref 955792 CRRT Dialysis System w/ Prismaflo II/S Heater
Baxter Prismaflex Ref 955792 CRRT Dialysis System w/ Prismaflo II/S Heater
Couldn't load pickup availability
The Baxter Prismaflex CRRT System is an advanced platform designed for continuous renal replacement therapy (CRRT) and other extracorporeal blood treatments in critical care. Buyers should be aware of the following capabilities:
-CRRT Therapy Modes – Supports multiple renal replacement modalities including CVVH (hemofiltration), CVVHD (hemodialysis), and CVVHDF (hemodiafiltration).
-Customizable Treatment – Allows therapy tailoring based on patient condition, with adjustable fluid balance and dose control.
-Integrated Scales – High-precision weighing system for input/output balance and accurate ultrafiltration control.
-Touchscreen Interface – Simple, menu-driven setup with guided treatment options for “New Patient,” “Same Patient,” and “Custom Mode.”
-Safety Features – Alarms for pressure monitoring, air detection, leaks, and scale deviations to help ensure patient safety.
-Prismaflo II/S Heater – Integrated external fluid warmer that maintains replacement/dialysate fluid at physiologic temperature.
-Compact and Mobile – Mounted on locking caster wheels for ease of movement within -clinical environments.
Condition / Testing Performed:
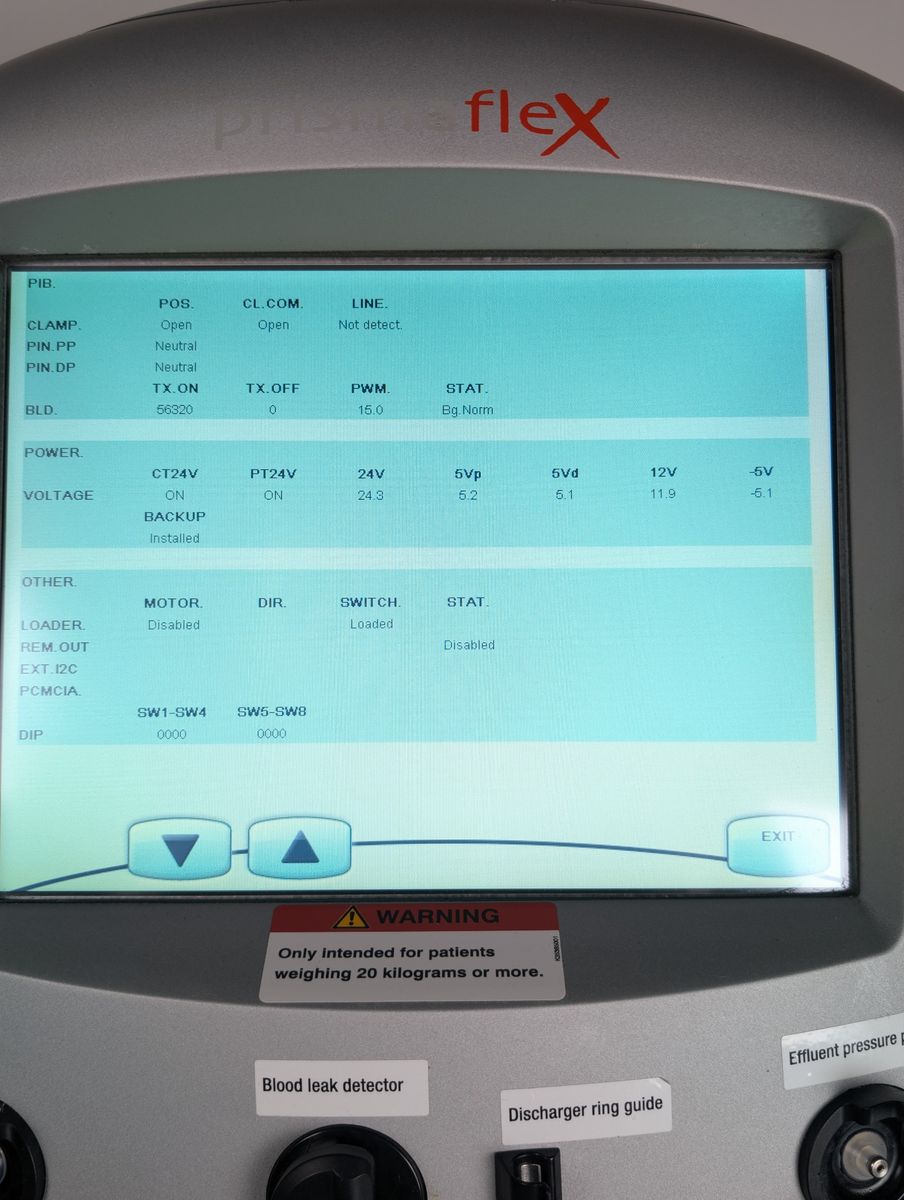
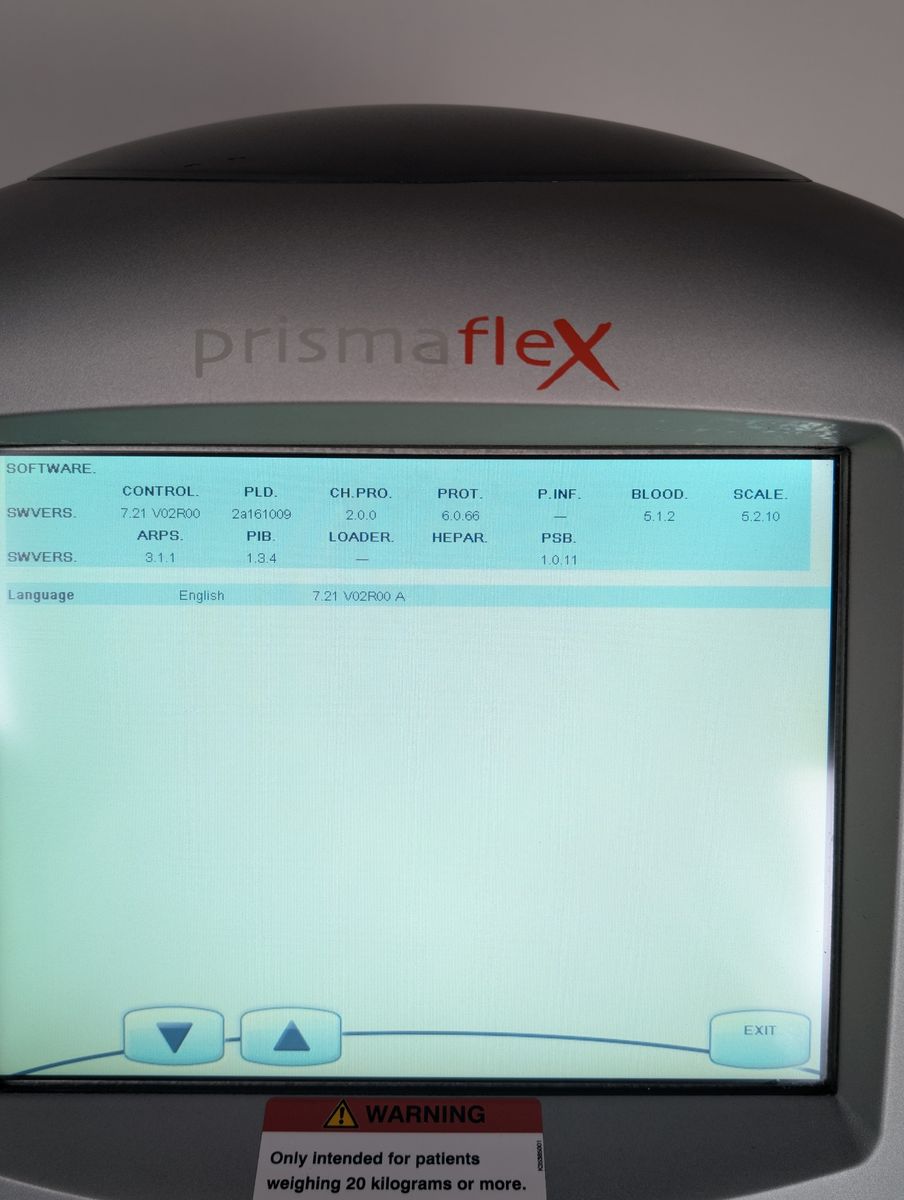
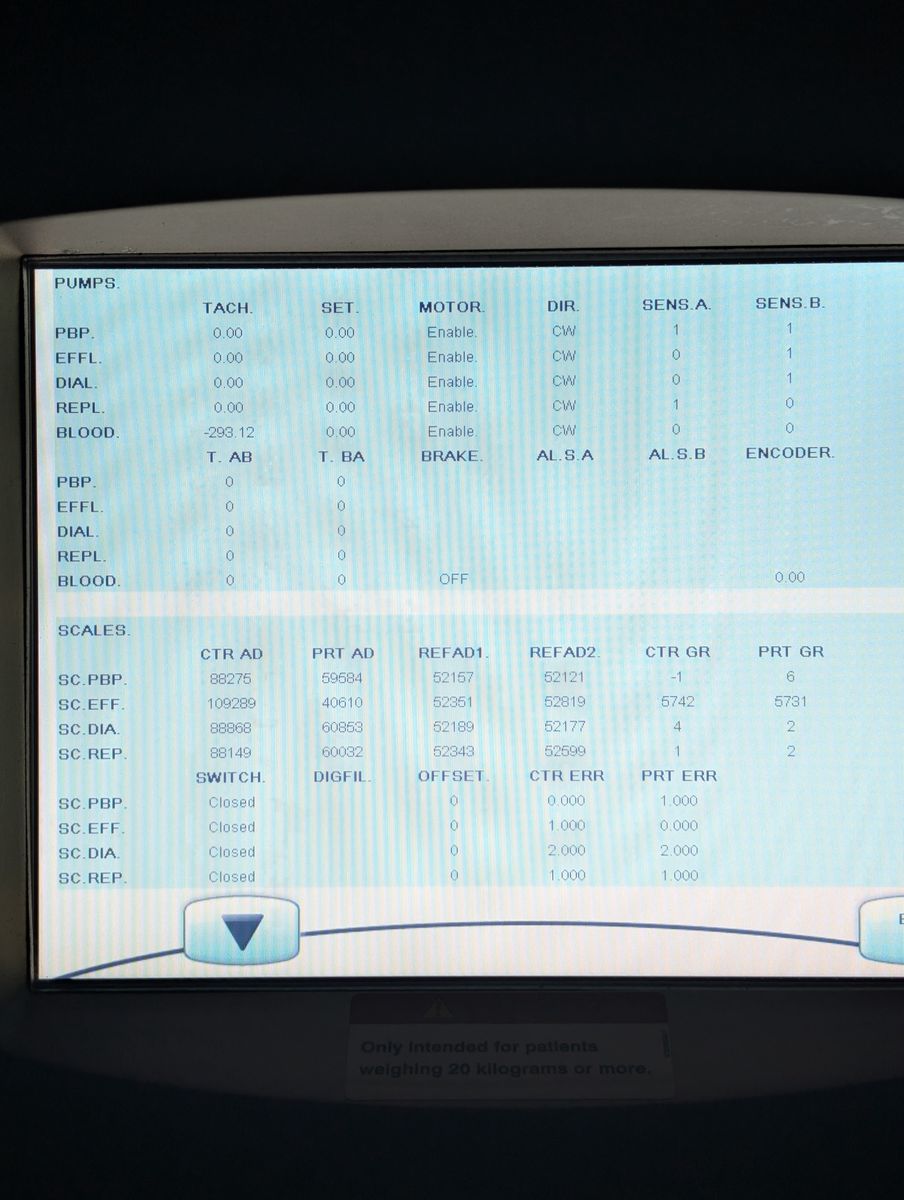
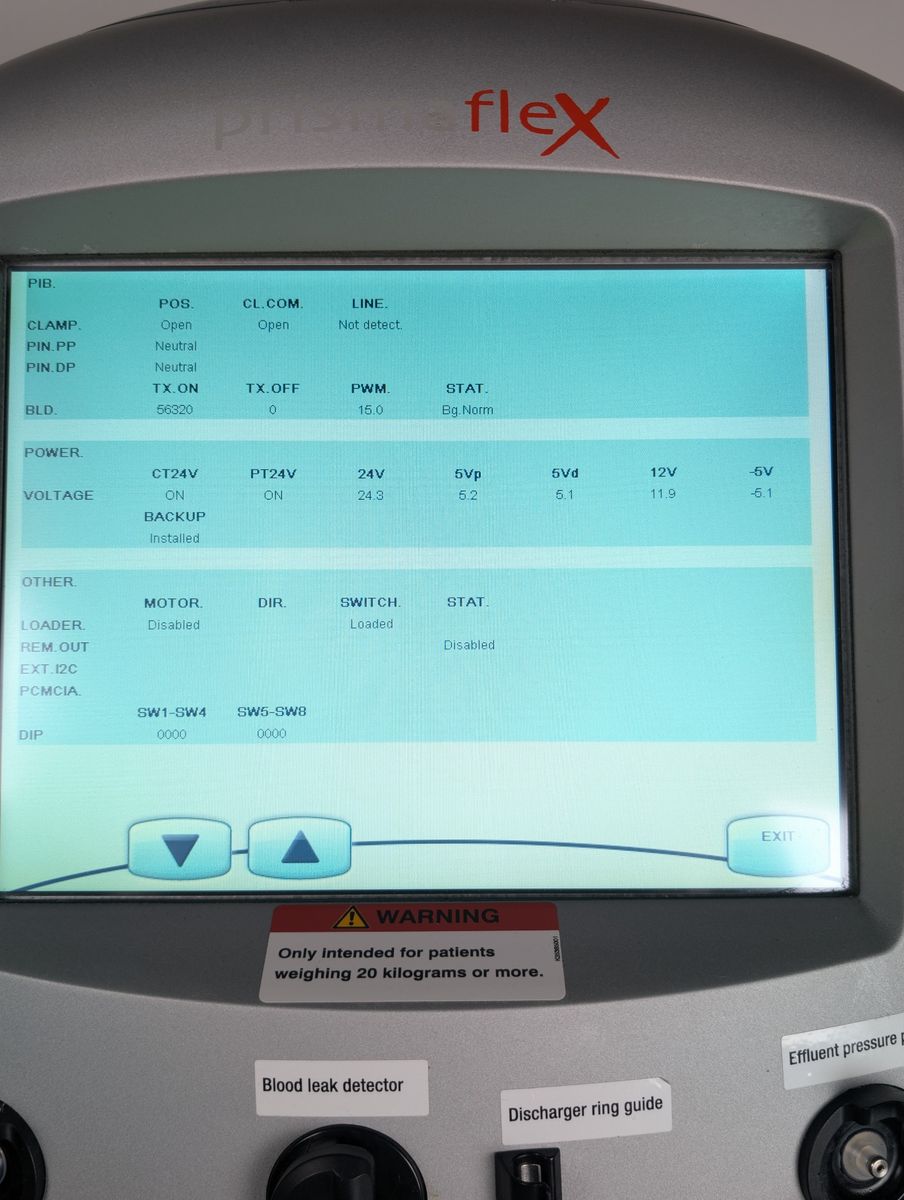
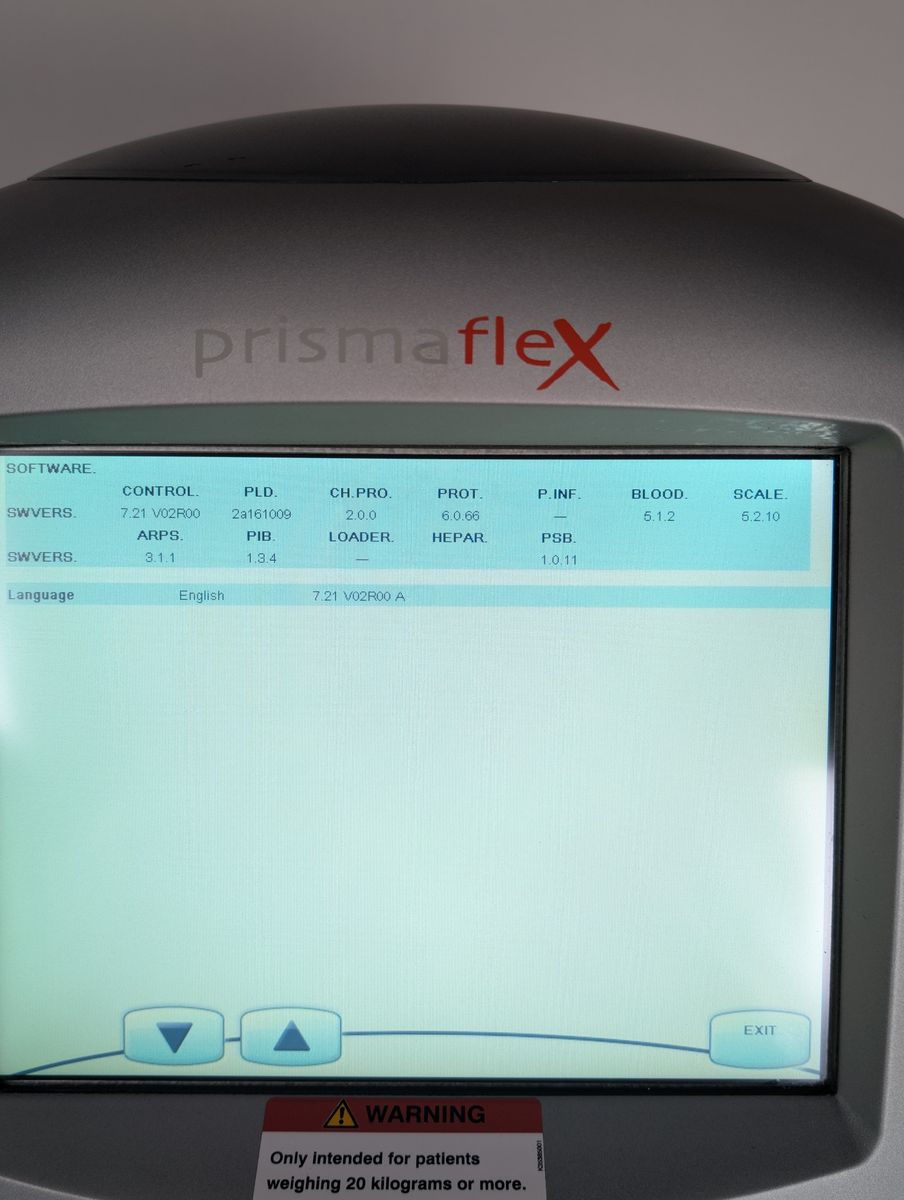
Unit powers on, boots to patient menu cleanly
Touchscreen responsive, menus navigable
Hour meter reads ~3018 hours
Preventive maintenance sticker shows last PM 7/2024, next due 7/2025
Prismaflo II/S heater powers on, responds to setpoint adjustment, and holds temperature (tested for 15+ minutes at 39.9°C)
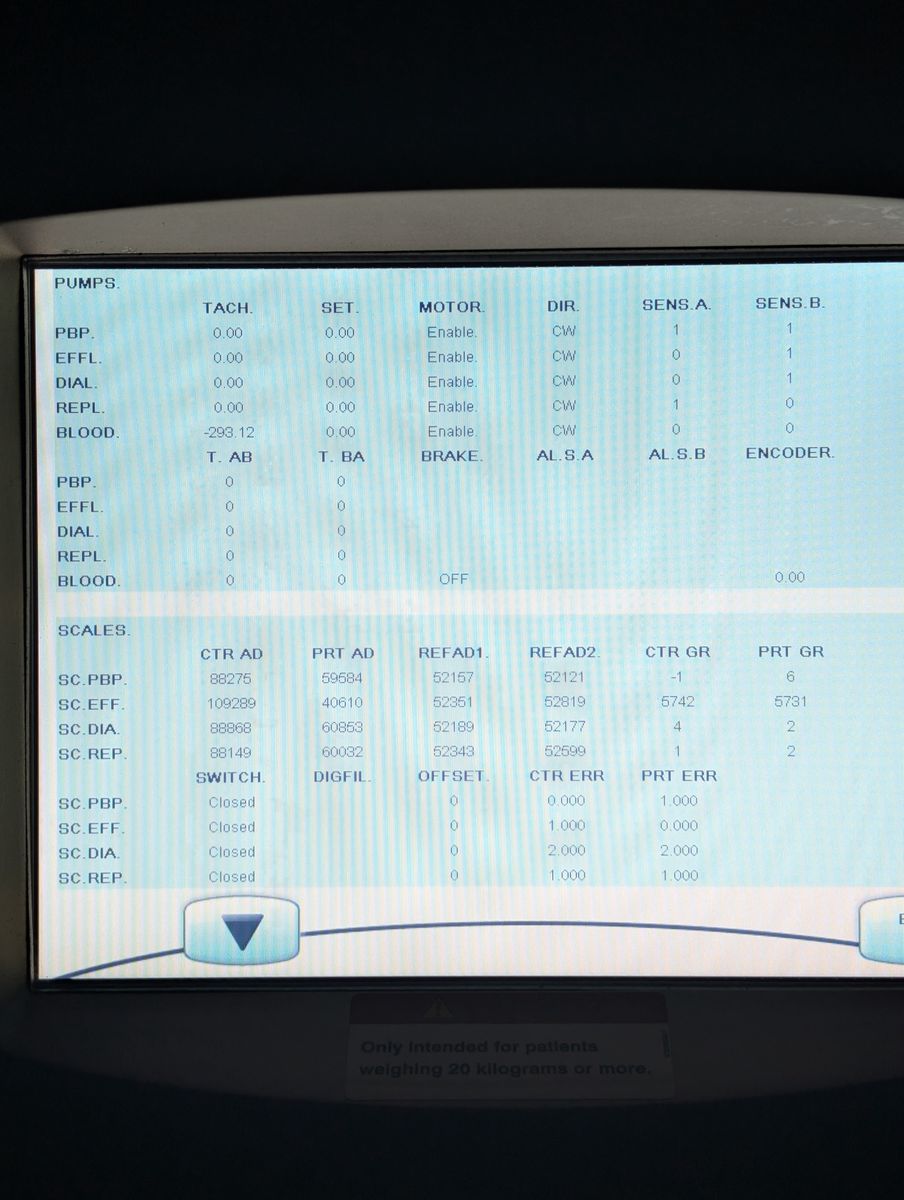
Bag holder scales seated properly; unit passed boot with no active scale errors
Caster wheels and locks tested, all function correctly
Included:
Baxter Prismaflex REF 955792 main unit
Prismaflo II/S heater REF PF2300NA
Heater hose REF PF2-WP33
Four bag holders
No disposables, cassettes, or additional accessories are included
Business Disclaimer
Garvis Med Equipment is a used medical equipment wholesaler. We do not service or refurbish any of the items that we sell and all items are sold used, as-is unless otherwise stated. We do not offer or imply any guarantees or warranties on any equipment sold. It is the responsibility of the buyer to ensure that all equipment is thoroughly inspected and refurbished by qualified technicians before placing into patient use. We accept no liability for equipment once it has left our warehouse.
FDA Regulatory Disclosure
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. Calibration and/or further testing might be necessary before use. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser before shipping. The item has been cleaned and handled in accordance with the manufacturer’s instructions.
Garvis Med Equipment
105 Clark St
Sellersburg, IN 47172
Phone: 812-565-9325
Rob Garvis
Share